What gives popping candy its signature explosive crackle
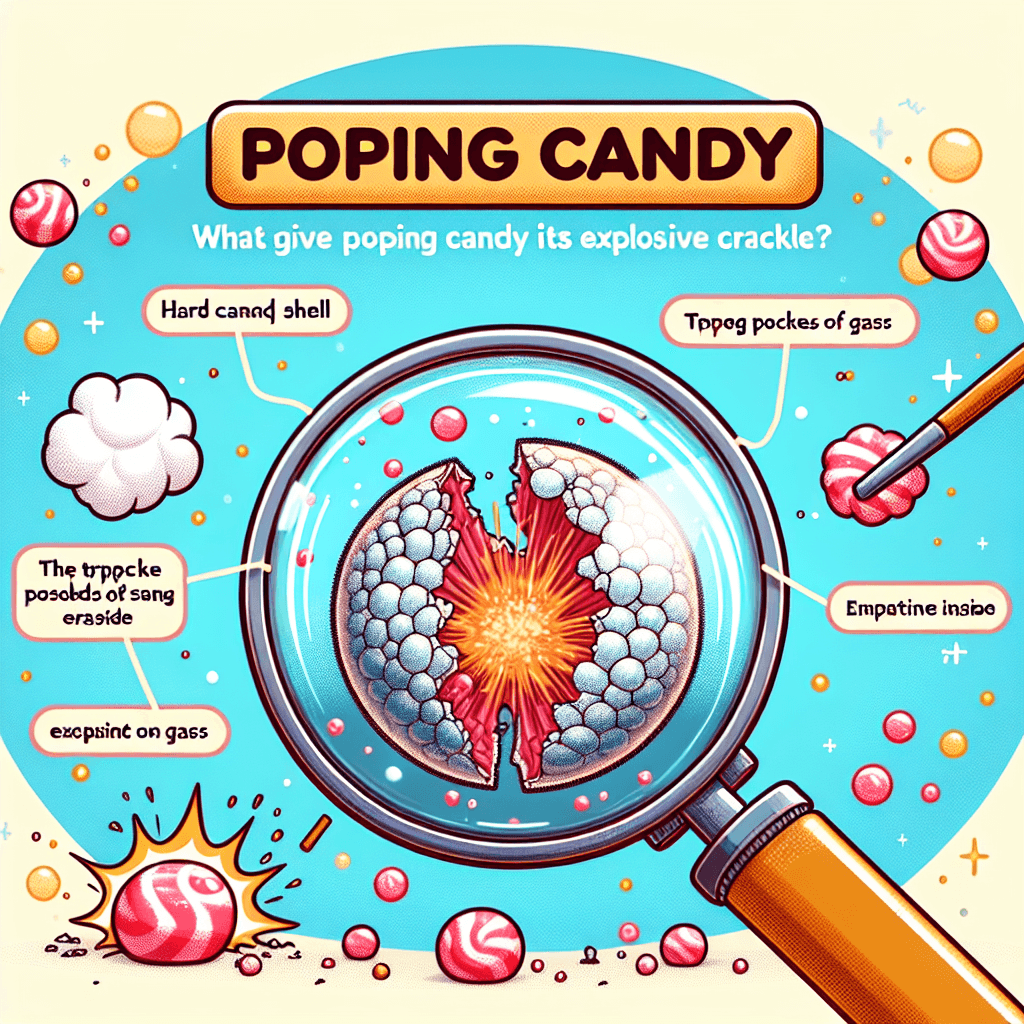
The secret isn't magic; it's a tiny, high-pressure explosion of gas trapped inside each crystal, just waiting to be released on your tongue.

Too Long; Didn't Read
TLDR: Popping candy has tiny, high-pressure bubbles of carbon dioxide gas trapped inside. When your saliva dissolves the candy, the gas escapes, causing the crackling and popping sounds.
The Science Behind the Snap: What Gives Popping Candy Its Signature Explosive Crackle?
Remember the first time you tipped a packet of popping candy into your mouth? That strange, delightful sensation of a tiny fireworks show on your tongue is a core memory for many. It’s a candy that you don't just taste, but also hear and feel. But what’s the secret behind this explosive treat? It’s not magic, but a fascinating piece of food science. This blog post will uncover the high-pressure secret trapped within each crystal, explaining exactly what gives popping candy its signature explosive crackle.
## The Secret Ingredient: Pressurized Gas
At its heart, the "pop" in popping candy is a simple, yet brilliant, physical reaction. The explosive crackle isn't caused by a chemical reaction, but by the release of a gas that has been trapped inside the candy under immense pressure. That gas is carbon dioxide (CO₂), the very same one that gives soda and other carbonated beverages their fizz.
Think of each piece of popping candy as a tiny, solid-form soda. Instead of being dissolved in liquid, the CO₂ is encased in a hard candy shell. When that shell dissolves, the gas escapes with an audible "pop," creating the tingling, crackling sensation that makes the candy so unique. The magic is simply the science of gas pressure in action.
## Forging the Fizz: The High-Pressure Manufacturing Process
Creating this unique confection requires a specialized process that forces the gas into the candy. It was invented by food chemist William A. Mitchell at General Foods in 1956, though it wasn't commercialized as Pop Rocks® until the mid-1970s. The process is a clever feat of food engineering.
Here’s a step-by-step look at how it’s made:
-
Creating the Candy Syrup: The process begins like many hard candies. A mixture of sugar, lactose, corn syrup, water, and flavorings is heated to a high temperature (around 300°F or 150°C) until it becomes a molten, liquid syrup.
-
The Carbonation Chamber: This is where the magic happens. The hot syrup is moved into a special pressurized vessel. This chamber is then flooded with carbon dioxide gas at an extremely high pressure—typically around 600 pounds per square inch (PSI). That's about 40 times the pressure of the air around us.
-
Trapping the Bubbles: The molten candy is mixed under this intense pressure, causing tiny bubbles of CO₂ to become trapped throughout the syrup.
-
Cooling and Shattering: The mixture is then allowed to cool while still under pressure. As the sugar solidifies, it hardens around these high-pressure gas bubbles, locking them in place. When the pressure in the chamber is finally released, the solid slab of candy shatters, breaking along the lines of the embedded gas pockets. This creates the small, irregular "rocks" that are then packaged and sold.
## The Final Pop: From Candy to Crackle
So, how does this carefully trapped gas finally make its escape? The release mechanism is your own saliva.
When you place the candy in your mouth, the moisture and warmth begin to dissolve the sugar and lactose matrix. As this sugary cage melts away, the walls of the tiny, pressurized bubbles become too thin to contain the CO₂. The gas bursts out, releasing its stored energy with a distinct pop and crackle. This sudden release of gas is what you feel as a fizzing sensation on your tongue. The more candy you eat, the more bubbles rupture, creating a symphony of tiny explosions.
Conclusion
The explosive crackle of popping candy is a testament to scientific ingenuity in the world of confections. It’s not a mysterious chemical, but pressurized carbon dioxide gas—the same fizz found in your favorite soda—trapped inside a hard candy shell. Through a meticulous process of heating, pressurizing, and cooling, manufacturers create tiny crystalline cages that hold this gas until the moment it dissolves in your mouth. So, the next time you enjoy this crackling treat, you can appreciate the clever food science that turns a simple sugar candy into an unforgettable sensory experience. It’s a perfect, and delicious, example of physics in action.


