Why do bubbles in a freshly poured soda first cling to the sides of the glass
It's no accident those bubbles cling to the glass—they're seeking out microscopic imperfections that act as tiny launchpads for their journey to the surface.

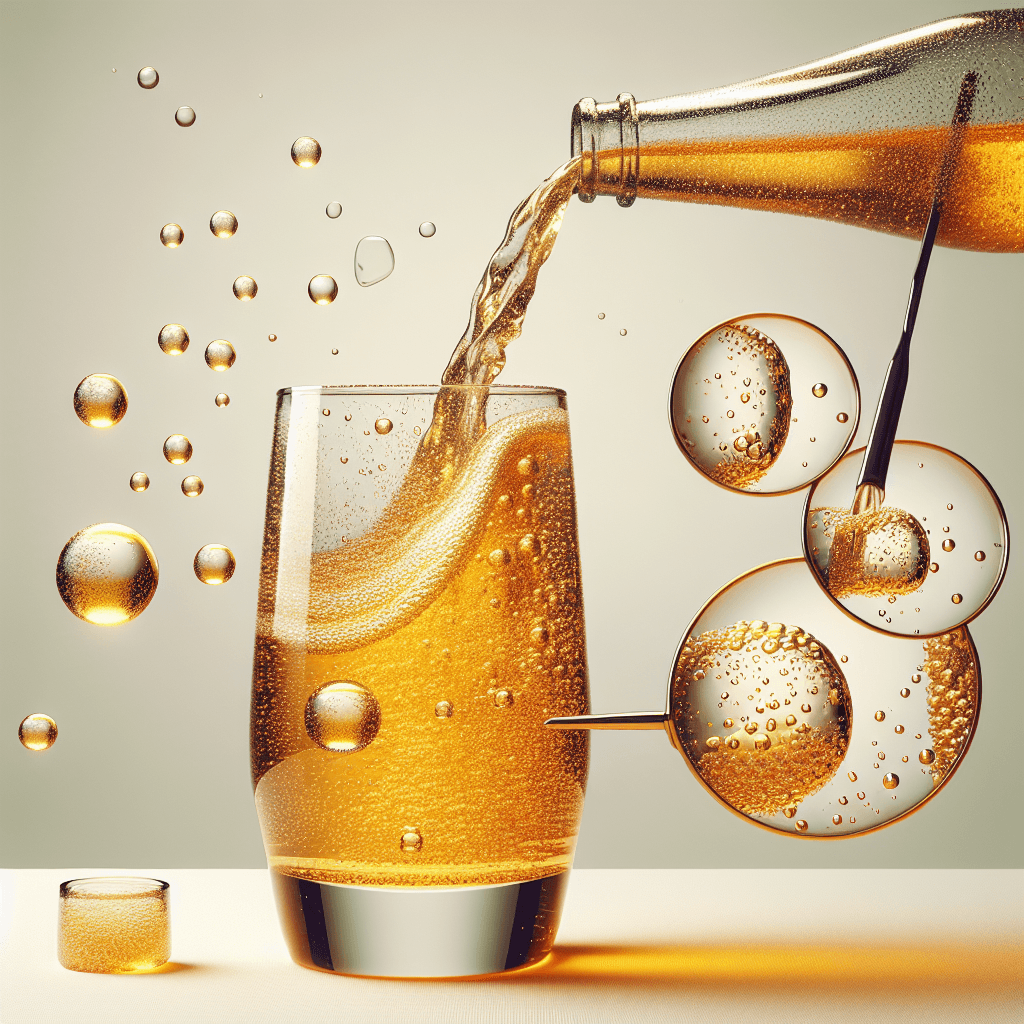
Too Long; Didn't Read
TLDR: Bubbles need a rough spot to form. Microscopic imperfections and dust particles on the sides of the glass act as perfect starting points for CO2 to escape the liquid, which is why bubbles cling there first.
The Science of Fizz: Why Do Bubbles in a Freshly Poured Soda First Cling to the Sides of the Glass?
Have you ever poured a cold, fizzy drink and taken a moment to watch the mesmerizing dance of bubbles? It’s a familiar sight: a cascade of effervescence rising to the surface. But before they begin their journey upward, many of those bubbles seem to appear from nowhere, clinging tenaciously to the sides of the glass. This isn't just a random occurrence; it's a fascinating display of physics at work in your kitchen. This common phenomenon reveals the secrets of how bubbles are born and why they behave the way they do. In this post, we'll dive into the science behind this bubbly behavior, exploring the invisible forces and microscopic imperfections that turn a simple glass of soda into a scientific spectacle.
The Secret Ingredient: Dissolved Carbon Dioxide
Before a bubble can form, its main ingredient needs to be present. The signature fizz in sodas, sparkling water, and champagne comes from carbon dioxide (CO2) gas. During the manufacturing process, a large amount of CO2 is dissolved into the liquid under high pressure and sealed in a can or bottle.
As long as the container is sealed, the pressure keeps the CO2 dissolved and invisible. However, the moment you open it, you hear that familiar psssst sound. This is the sound of the pressure being released. Now, the CO2 is in a "supersaturated" state, meaning there is more gas dissolved in the liquid than it can naturally hold at normal atmospheric pressure. The gas wants to escape, and it does so by forming bubbles.
The Birth of a Bubble: Unmasking Nucleation Sites
A bubble needs a starting point—a place to be born. In scientific terms, this starting point is called a nucleation site. It is far more difficult for CO2 molecules to spontaneously group together in the middle of a perfectly smooth liquid to form a bubble. They need a tiny, pre-existing pocket of gas or an irregularity to gather around. It’s the difference between building a structure on a flat, empty field versus building it on a pre-laid foundation.
This is where the surface of your glass comes in. To the naked eye, a clean glass appears perfectly smooth. On a microscopic level, however, it’s a rugged landscape of pits, scratches, and crevices. In addition to these imperfections in the glass itself, other nucleation sites can include:
- Tiny, trapped air pockets.
- Microscopic fibers left over from a cleaning cloth.
- Small specks of dust or other impurities.
These tiny imperfections are the perfect incubators for bubbles.
Why the Sides? The Role of Surface Imperfections
When you pour your soda, the liquid rushes in and traps microscopic pockets of air within these tiny cracks and crevices on the glass wall. These trapped air pockets are the ideal nucleation sites.
The dissolved CO2 molecules, eager to escape the liquid, find these air pockets and begin to collect there. As more and more CO2 molecules accumulate, the pocket grows until it becomes large and buoyant enough to detach from the side of the glass and float to the surface. This is why you see streams of bubbles originating from the same, seemingly invisible spots on the glass—you're witnessing active nucleation sites at work. A perfectly smooth glass with no imperfections would, in theory, produce far fewer bubbles.
This principle is famously used in some champagne flutes, which have a tiny, laser-etched point at the bottom. This intentional imperfection acts as a perfect, single nucleation site, creating a beautiful, continuous stream of bubbles from the center of the glass.
Conclusion
So, the next time you pour a fizzy drink, you’ll know that the bubbles clinging to the sides aren’t just resting there. They are being actively formed in the microscopic nooks and crannies of the glass. This phenomenon is a brilliant, everyday example of nucleation—the process where dissolved carbon dioxide finds an imperfection, gathers its strength, and forms a bubble to make its escape. It’s a reminder that even in the most mundane observations, there is a world of fascinating science waiting to be discovered. Take a closer look; you're not just enjoying a refreshing beverage, you're watching physics in action.