Why does biting down on aluminum foil sometimes cause a sharp jolt of pain
That cringeworthy, electric jolt isn't your imagination—it's the shocking result of you accidentally creating a tiny battery inside your own mouth.

Too Long; Didn't Read
TLDR: If you have metal fillings, biting on foil creates a tiny battery in your mouth using your saliva as a conductor. This makes a small electric current that shocks the nerve in your tooth, causing a jolt of pain.
The Shocking Truth: Why Does Biting Down on Aluminum Foil Sometimes Cause a Sharp Jolt of Pain?
Have you ever taken a bite of a leftover sandwich or a piece of chocolate wrapped in foil, only to be met with a sudden, sharp, metallic-tasting jolt of pain that seems to come from nowhere? It’s a startling and deeply unpleasant sensation that can make you wince. You might have wondered if you were imagining it or if something was seriously wrong with your tooth. The good news is that you're not alone, and the experience isn't dangerous. This bizarre phenomenon is a perfect, real-world example of basic chemistry happening inside your mouth. This post will break down the science behind this oral shock, explaining exactly why that innocent piece of foil can turn your mouth into a tiny, pain-inducing battery.
It’s All About a Galvanic Shock
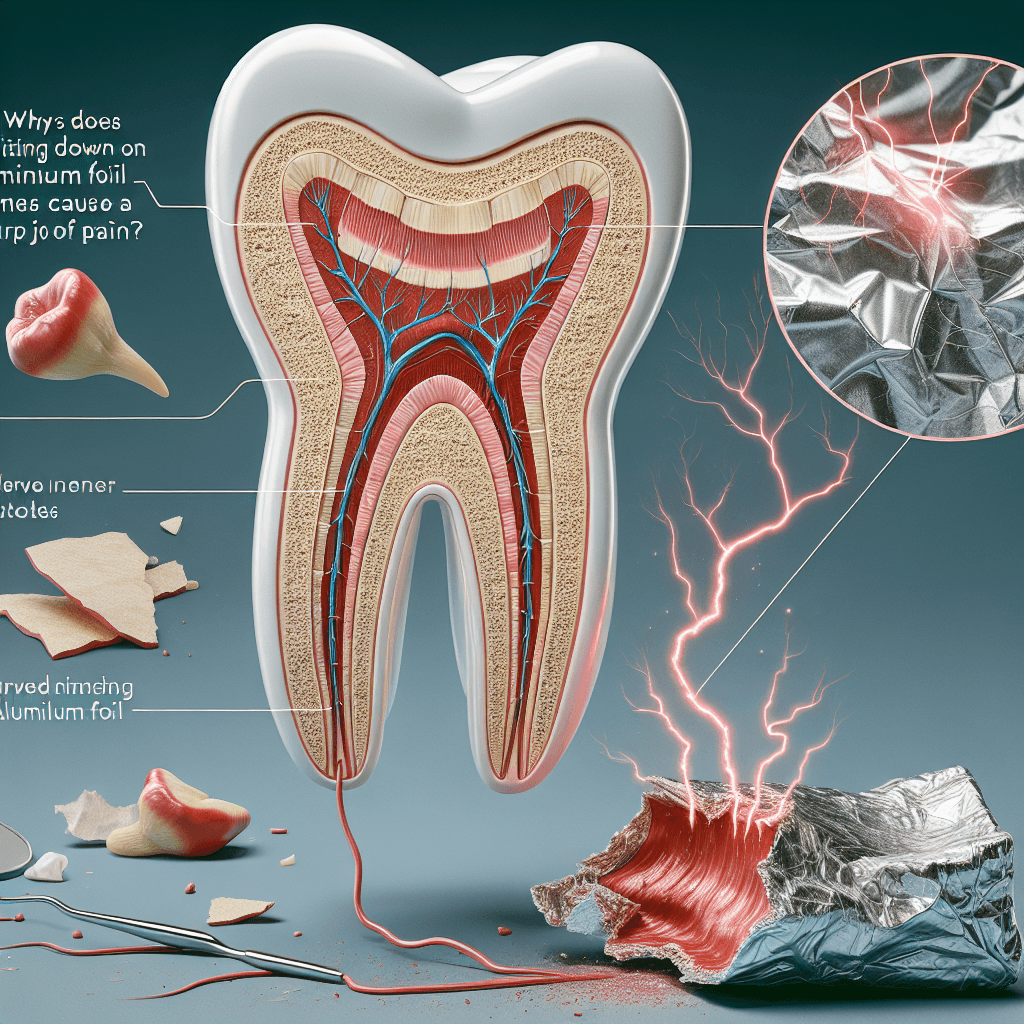
The sharp pain you feel from biting on aluminum foil is a well-documented phenomenon known as a galvanic shock or galvanic corrosion. At its core, this is an electrochemical process—the same principle that powers a simple battery. For a battery to work, you need three key components: two different metals and a conductive solution called an electrolyte.
When you bite on a piece of foil, your mouth can suddenly provide all three of these components:
- Metal #1: The aluminum foil.
- Metal #2: A metal dental filling, such as a silver amalgam filling (which is a mix of mercury, silver, tin, and copper) or a gold crown.
- The Electrolyte: Your saliva. The salts and other compounds in your saliva make it an excellent conductor of electricity.
When these three elements come together, they create a complete electrical circuit, and your mouth momentarily becomes a tiny, low-voltage battery.
How the Shock Happens: A Step-by-Step Breakdown
The process unfolds in a fraction of a second, but it involves a fascinating chain of events. The key is that different metals have different electrical potentials. Aluminum is a highly reactive metal compared to the more noble metals found in dental fillings like gold or the silver-tin alloy in amalgam.
- The Metals Connect: When you bite down, the aluminum foil physically touches your metal filling.
- The Saliva Bridge: Your saliva acts as a bridge, connecting the two different metals and allowing electrons to flow between them.
- An Electric Current is Born: Because of the difference in electrical potential, electrons are stripped from the more reactive aluminum atoms and flow toward the less reactive metal in your filling. This movement of electrons is, by definition, an electric current.
- The Nerve Gets Zapped: This tiny but potent electrical current travels through the metal filling, which is an excellent conductor. The current makes its way down into the very sensitive pulp of your tooth, where the nerve endings are located. Your dental nerve isn't designed to interpret this type of stimulation, so it registers the electrical signal as a sudden, sharp jolt of pain.
Do You Need a Filling to Feel the Zap?
For the most part, yes. This experience is almost exclusive to people who have metallic dental restorations. If you have only composite (tooth-colored) fillings or have never had a cavity, biting on foil is unlikely to cause anything more than an unpleasant texture. Without a second, different type of metal in your mouth, the "battery" circuit cannot be completed, and no electric current will be generated. The greater the difference in reactivity between the two metals—for instance, between aluminum foil and a gold crown—the stronger the potential voltage and the more intense the shock can be.
Is This Jolt Harmful?
While the sensation is alarming and certainly painful, a galvanic shock is completely harmless. The electric current is incredibly small and lasts only as long as the two metals are in contact through the saliva. It causes no lasting damage to your tooth, your filling, or your overall health. The only real "danger" is the momentary, unpleasant shock itself. The best way to deal with it is simply to avoid it—be careful when eating foods that might have small pieces of foil on them.
Conclusion
That shocking jolt from biting on aluminum foil is not a sign of a serious dental problem but rather a fascinating lesson in electrochemistry. By combining two different metals (the foil and a filling) with an electrolyte (your saliva), you inadvertently create a simple battery in your mouth. The resulting electric current stimulates your tooth’s nerve, causing that memorable zap of pain. It’s a powerful reminder that science is happening all around us—and sometimes, even inside our own mouths. So, the next time you unwrap a baked potato or a piece of candy, take a moment to ensure all the foil is gone. Your dental nerves will thank you for it.


