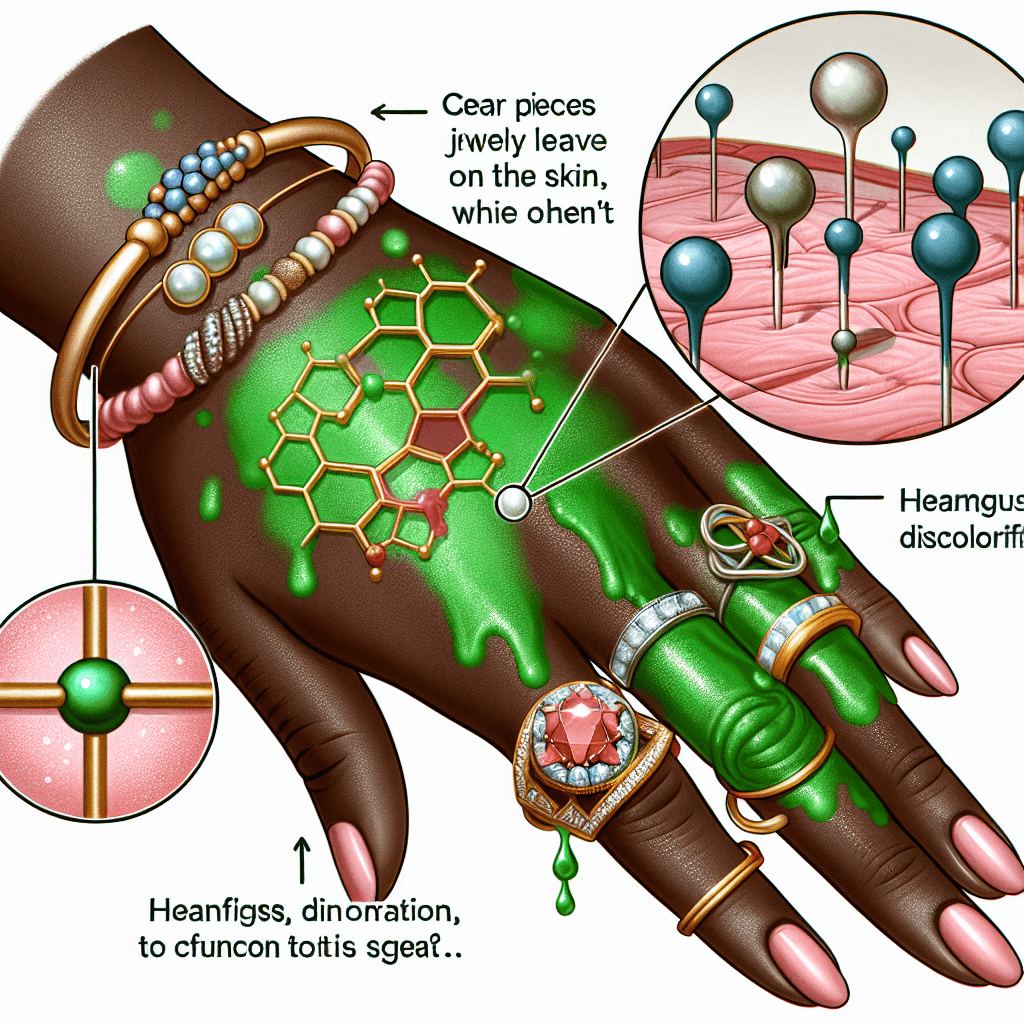
Why does some jewelry leave a green stain on your skin while other jewelry does not
That green mark on your finger isn't an allergy or a sign of "fake" jewelry; it's a fascinating chemical reaction happening right on your skin.

Too Long; Didn't Read
TLDR: The green stain is a harmless chemical reaction, not an allergy. Copper in the jewelry reacts with the acids in your sweat and lotions, causing it to oxidize and leave a green film on your skin. Jewelry made of non-reactive metals like platinum, titanium, or high-karat gold won't stain because they contain little to no copper.
The Green Stain Mystery: Why Does Some Jewelry Leave a Green Stain on Your Skin While Other Jewelry Does Not?
You take off your favorite ring at the end of a long day, only to find a faint, greenish-black mark left behind on your finger. Is it an allergic reaction? Is the jewelry fake? More importantly, is it harmful? This experience is surprisingly common, and the explanation has more to do with basic chemistry than the quality of your accessory. While that green stain can be alarming, it’s rarely a cause for concern. This post will delve into the science behind why some jewelry leaves a green mark, which metals are the usual culprits, and what you can do to prevent it, ensuring you can wear your favorite pieces with confidence.
The Science Behind the Green: It's All About Oxidation
That dreaded green stain is not a sign of an allergy or an infection. It's the result of a simple chemical reaction between the metal in your jewelry and your skin. The primary culprit behind this discoloration is copper.
When copper is exposed to moisture, salts, and acids—all of which are present in your sweat and on your skin—it begins to oxidize. This process creates a layer of copper salts (like copper carbonate), which are blue-green in color. These compounds then rub off and are absorbed by the top layers of your skin, leaving behind a temporary stain. Think of it as a miniature version of the same chemical reaction that gives the copper-clad Statue of Liberty its famous green patina. This reaction is completely harmless and the stain will typically fade on its own within a day or two.
Common Jewelry Metals That Can Cause Staining
You might be surprised to learn that even high-quality jewelry can contain copper. Pure metals are often too soft for everyday wear, so they are mixed with other metals to create stronger, more durable alloys.
- Sterling Silver: By definition, sterling silver is an alloy made of 92.5% pure silver and 7.5% other metals, most commonly copper. The presence of copper is what makes sterling silver strong enough to be crafted into intricate designs, but it also means it can tarnish and potentially leave a green or black mark on the skin, especially in humid conditions or for individuals with more acidic skin chemistry.
- Lower-Karat Gold: Pure gold (24 karat) is extremely soft and non-reactive. However, it's rarely used in jewelry because it bends and scratches easily. To increase its durability, gold is alloyed with other metals like silver, zinc, and copper. In lower-karat gold, such as 14k or 10k, the percentage of these other metals is higher. Rose gold, for example, gets its beautiful pinkish hue from a high copper content, making it a more likely candidate for causing a green stain.
- Brass and Bronze: These metals are very common in fashion and costume jewelry. Brass is an alloy of copper and zinc, while bronze is an alloy of copper and tin. Since copper is a primary component of both, jewelry made from these materials is highly likely to cause skin discoloration.
The "Safe" Metals: Why Some Jewelry Never Stains
If some metals stain, why don't all of them? The answer lies in their chemical stability. Certain metals are highly resistant to oxidation and corrosion, making them excellent choices for those who want to avoid the green-skin effect.
These non-reactive or hypoallergenic metals include:
- Platinum: An extremely durable and dense precious metal that is highly unreactive.
- Titanium: A lightweight, strong, and biocompatible metal that will not corrode or react with skin.
- Surgical-Grade Stainless Steel: An iron-based alloy that is highly resistant to rust, tarnish, and corrosion.
- High-Karat Gold (18k and above): These pieces contain a higher percentage of pure gold and a lower percentage of other alloys like copper, making them far less likely to react with your skin.
- Rhodium-Plated Jewelry: Many sterling silver and white gold pieces are plated with a thin layer of rhodium (a member of the platinum family). This plating provides a bright, tarnish-resistant finish and acts as a protective barrier between your skin and the copper-containing alloys underneath. However, this plating can wear off over time.
Is the Green Stain Harmful? Plus, Prevention Tips
For the vast majority of people, the green stain is entirely harmless. It is simply a surface discoloration, not a sign of a true metal allergy, which would typically involve symptoms like redness, itching, swelling, or a rash.
If you love a piece of jewelry that tends to leave a mark, here are a few simple tips to prevent it:
- Keep It Dry: Remove your jewelry before washing your hands, showering, swimming, or exercising.
- Avoid Chemicals: Apply lotions, perfumes, and soaps before you put your jewelry on, and make sure they are fully absorbed.
- Clean Your Jewelry: Regularly clean your pieces with a soft cloth to remove residue and oils that can accelerate oxidation.
- Create a Barrier: For rings or bracelets, you can apply a thin coat of clear nail polish to the inside surface that touches your skin. This creates a temporary barrier, but it will need to be reapplied periodically.
Conclusion
Seeing a green stain on your skin can be unsettling, but it's rarely anything more than a bit of simple chemistry in action. The reaction of copper in metal alloys with the elements on your skin is a natural and harmless process. Understanding the composition of different metals not only solves the mystery of the green stain but also empowers you to make more informed choices when purchasing your next piece of jewelry. So, the next time you see that faint green hue, you'll know it's just science, not a sign of trouble, allowing you to enjoy your beautiful accessories without worry.