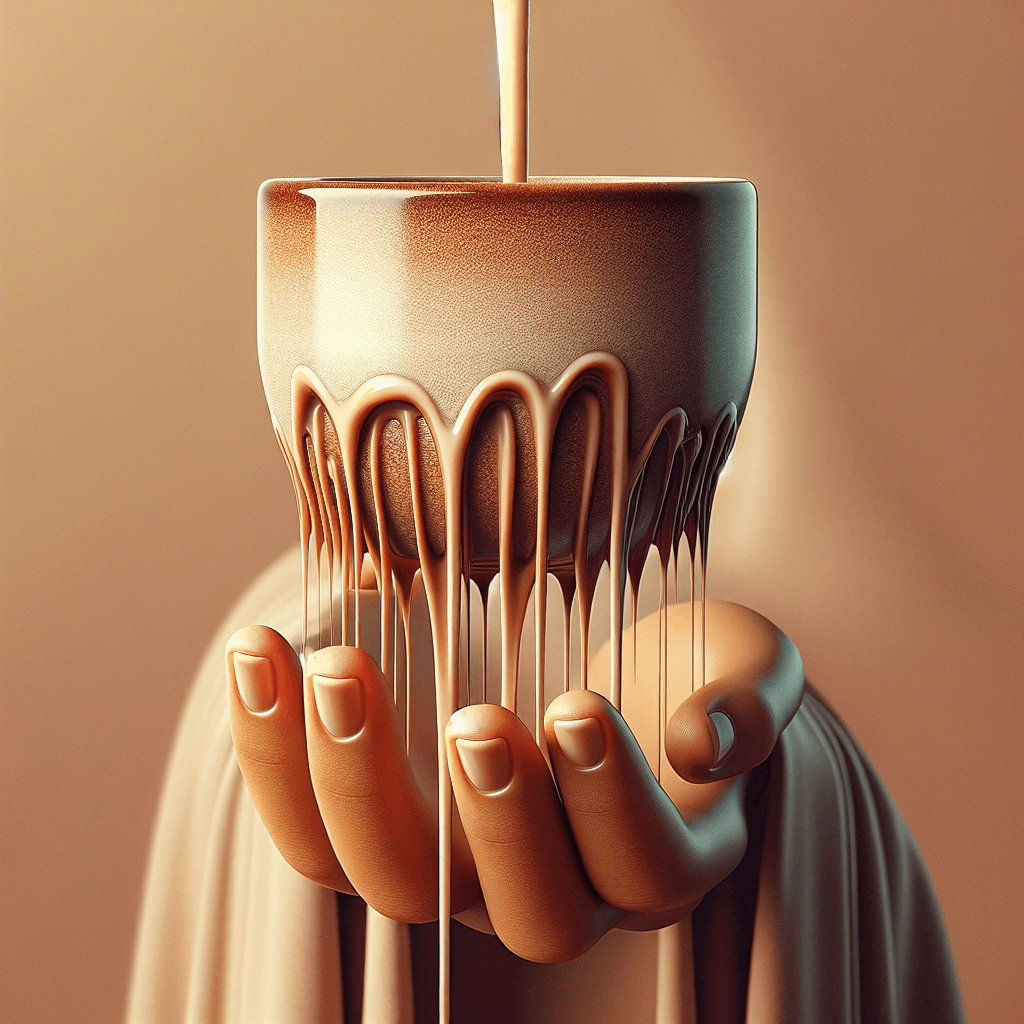
What makes liquid dribble down the side of a cup when you try to pour slowly
It’s the infuriating moment when liquid defies gravity and clings to the cup, and the simple law of physics behind this universal frustration is truly mind-bending.

Too Long; Didn't Read
TLDR: Liquid naturally clings to the side of the cup. When you pour slowly, there is not enough momentum to break that cling, causing it to dribble. Pouring faster provides the force needed for a clean pour.
The Teapot Effect Explained: What Makes Liquid Dribble Down the Side of a Cup When You Pour Slowly?
We’ve all been there. You’re carefully trying to pour a cup of coffee for a guest, or perhaps just topping off your glass of water, and you decide to pour slowly and deliberately. Instead of a clean stream, a rogue trickle of liquid defies gravity, clings to the side of the cup, and creates a messy puddle on your counter. This frustratingly common phenomenon isn’t a sign of a clumsy pourer; it's a perfect, everyday demonstration of some fascinating physics.
This blog post will demystify this kitchen nuisance, known scientifically as the "Teapot Effect." We'll explore the invisible forces at play that cause this dribbling and explain why a little confidence in your pour can make all the difference.
Meet the Main Culprit: The Teapot Effect
The tendency for a liquid to follow a curved surface rather than falling straight down is officially called the Teapot Effect, a specific manifestation of a broader fluid dynamics principle known as the Coanda effect. First described by Romanian inventor Henri Coandă, this effect explains how a jet of fluid—whether a gas or a liquid—tends to be "attracted" to a nearby surface and follow its curve.
Think of how air flows over the curved top of an airplane wing to generate lift; that's the Coanda effect in action with a gas. When you pour slowly from a cup with a thick, rounded lip, you're creating the perfect conditions for the same principle to take over, but with your drink.
The Tug-of-War: A Trio of Scientific Forces
The Teapot Effect doesn't happen in a vacuum. It’s the result of a delicate battle between several molecular forces. When you pour slowly, you give the "clinging" forces the upper hand.
Adhesion and Cohesion
At the heart of the matter are two competing forces:
- Adhesion: This is the force of attraction between molecules of different substances. In this case, it’s the attraction between the liquid molecules (like water or coffee) and the molecules of the cup's material (like ceramic or glass).
- Cohesion: This is the force of attraction between molecules of the same substance. It's what holds a droplet of water together.
When you pour slowly, the stream of liquid makes contact with the lip of the cup. The force of adhesion is strong enough to "grab" the leading edge of the stream and pull it slightly toward the cup's surface.
Surface Tension
Working alongside cohesion is surface tension. This is the tendency of a liquid to shrink into the minimum surface area possible, creating a sort of thin "skin" on its surface. When adhesion pulls part of the stream onto the cup’s lip, surface tension ensures the rest of the stream follows suit, pulling the entire flow down the side of the vessel instead of away from it.
It's Not You, It's the Cup (And Your Speed)
Now that we understand the forces, it becomes clear why certain factors make the dribbling worse.
-
Pouring Speed: When you pour slowly, the liquid's momentum is low. This allows the relatively weak forces of adhesion and surface tension to win the tug-of-war and redirect the stream. When you pour quickly and confidently, the liquid’s momentum is much higher, easily overcoming adhesion and shooting straight out from the lip.
-
Cup and Spout Design: The shape of the pouring edge is critical.
- Thick, Rounded Lips: A standard coffee mug is the worst offender. Its thick, rounded lip provides a large, smooth, curved surface, creating an ideal pathway for the Coanda effect to take hold.
- Sharp, Drip-Free Edges: A pitcher or scientific beaker, by contrast, often has a sharp, defined spout. This sharp edge acts as a point of separation. It effectively breaks the surface tension and doesn't give adhesion a smooth path to follow, forcing the liquid to detach cleanly. This is why a well-designed teapot, ironically, is one of the best vessels for avoiding the Teapot Effect.
Conclusion
That frustrating dribble down the side of your cup is more than just a mess; it’s a beautiful, real-world lesson in fluid dynamics. It's the result of a delicate interplay between adhesion, cohesion, and the liquid’s own momentum, all governed by the Coanda effect. The molecular attraction between your drink and your cup is literally pulling the stream astray.
So, the next time you go to pour, remember the science. The solution isn't to be timid; it's to pour with a bit of speed and confidence. By doing so, you give the liquid's momentum the advantage it needs to overcome those clingy molecular forces, ensuring your drink lands exactly where it belongs: inside the other cup.