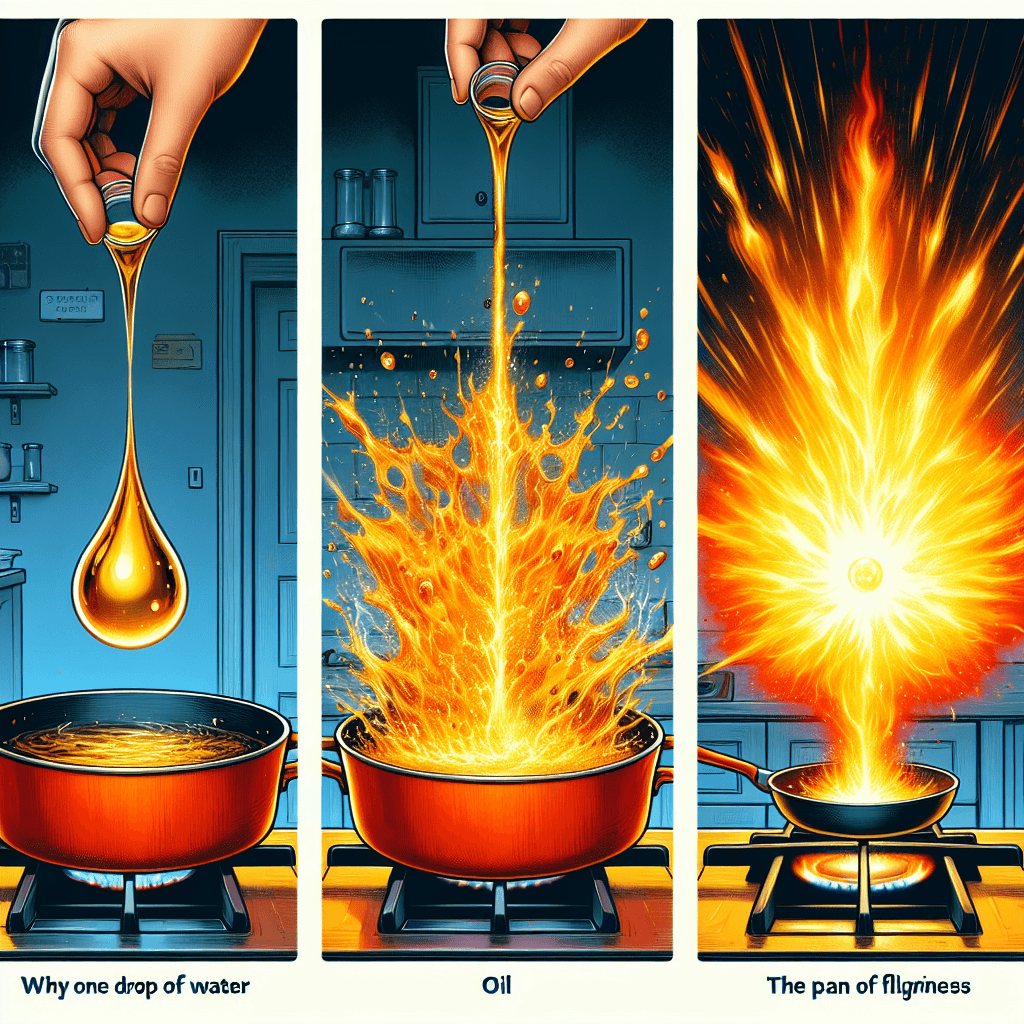
Why can one drop of water make a pan of hot oil erupt in flames
That tiny drop of water doesn't just sizzle; it instantly flash-boils into a miniature steam explosion that can launch a geyser of flaming oil right in your kitchen.

Too Long; Didn't Read
TLDR: Water sinks in hot oil and instantly flash-boils into steam, expanding violently. This explosion launches a fine mist of oil into the air, which then has enough surface area to ignite into a massive fireball.
The Kitchen Catastrophe: Why Can One Drop of Water Make a Pan of Hot Oil Erupt in Flames?
It’s a scene many of us have witnessed: a stray drop of water falls into a pan of sizzling-hot oil, and the result is a violent crackle and spit. But what happens when that pan is exceptionally hot? That harmless sizzle can transform into a terrifying eruption of flames. This isn't a freak accident; it's a predictable, and preventable, result of basic physics and chemistry. Understanding why this happens is not just a fascinating science lesson—it's a critical piece of knowledge for anyone who cooks. This post will break down the science behind this dangerous kitchen phenomenon, explaining exactly why water and hot oil are a recipe for disaster.
The Clash of Temperatures: Water vs. Oil
The entire violent reaction begins with a fundamental difference between water and cooking oil: their boiling points. Water, as we all know, boils at 212°F (100°C). Cooking oils, however, are a different story. The temperature at which you typically fry food is between 350°F and 375°F (175°C to 190°C), well above water's boiling point. If the oil gets hot enough to catch fire on its own (its flash point), it can be upwards of 600°F (315°C).
When a drop of water, which is denser than oil, enters the pan, it sinks beneath the surface. There, it is instantly exposed to temperatures hundreds of degrees hotter than its boiling point. This extreme temperature difference is the trigger for a rapid and violent chain reaction.
From Sizzle to Explosion: The Rapid Expansion
The heart of the eruption lies in an incredible physical transformation. When the submerged water droplet is superheated by the oil, it doesn't just boil—it flash-vaporizes into steam.
The key fact here is the sheer scale of the expansion. According to fundamental principles of thermodynamics, when liquid water converts to steam, it expands to approximately 1,700 times its original volume. This instantaneous, massive expansion acts like a small explosion at the bottom of the pan. This steam explosion violently ejects everything above it—namely, the scalding hot oil—upwards and outwards in a spray of tiny droplets.
The Final Ingredient: Atomization and Ignition
This is where the situation escalates from a dangerous mess to a full-blown fire. The steam explosion doesn't just push the oil out of the pan; it atomizes it. This means the oil is broken up into a fine, aerosolized mist. This process dramatically increases the oil's surface area, making it incredibly easy to ignite.
This cloud of flammable oil mist is then propelled upwards, where it meets the heat source responsible for the fire in the first place—either a gas flame or a glowing-hot electric stovetop element. With a massive surface area, plenty of oxygen in the air, and a direct ignition source, the oil mist instantly combusts, creating the terrifying fireball that can engulf a kitchen in seconds. It’s not the pan of oil itself that erupts in flame, but the cloud of atomized oil above it.
How to Safely Handle a Grease Fire
Knowing the science underscores the most important rule of kitchen safety: NEVER use water to put out a grease fire. Doing so will only trigger the explosive reaction described above, turning a contained fire into an uncontrollable inferno. Instead, if you face a grease fire, follow these steps:
- Turn Off the Heat: Immediately cut the power to the stove. Don't try to move the pan.
- Smother the Flames: Cut off the fire's oxygen supply by sliding a metal lid or a baking sheet over the pan. Do not use a glass lid, which can shatter.
- Use Baking Soda: For a small fire, you can douse it with a large amount of baking soda, which releases carbon dioxide and smothers the fire. Never use flour, which is combustible.
- Use a Fire Extinguisher: A Class K fire extinguisher is designed for kitchen fires, but a Class B (for flammable liquids) will also work.
Conclusion
The terrifying eruption of a pan of hot oil is not a mystery, but a predictable three-step process: the superheating of water, its explosive expansion into steam, and the subsequent ignition of the atomized oil. A single drop of water contains the potential for a catastrophe because it acts as the propellant for the highly flammable fuel. This knowledge is your best defense. By understanding the science behind the sizzle, you can ensure your kitchen remains a place of creation, not combustion, and keep yourself and your home safe from the devastating consequences of a grease fire.


